Our company has extensive expertise in providing services for the expression and purification of recombinant proteins. In order to provide you with multiple options for protein expression and purification, our researchers have developed specialized expression services for yeast systems. Our company has established a sophisticated yeast expression service platform to provide expression and purification of high-quality recombinant proteins in Pichia pastoris.
Backgrounds
The yeast expression system has the advantages of both prokaryotic and advanced eukaryotic systems. It has common culture conditions, a fast growth rate, relatively low cost, ability to perform post-translational processing of proteins, and easy access to soluble active recombinant proteins. It is widely used in the production and preparation of recombinant proteins, especially eukaryotic proteins.
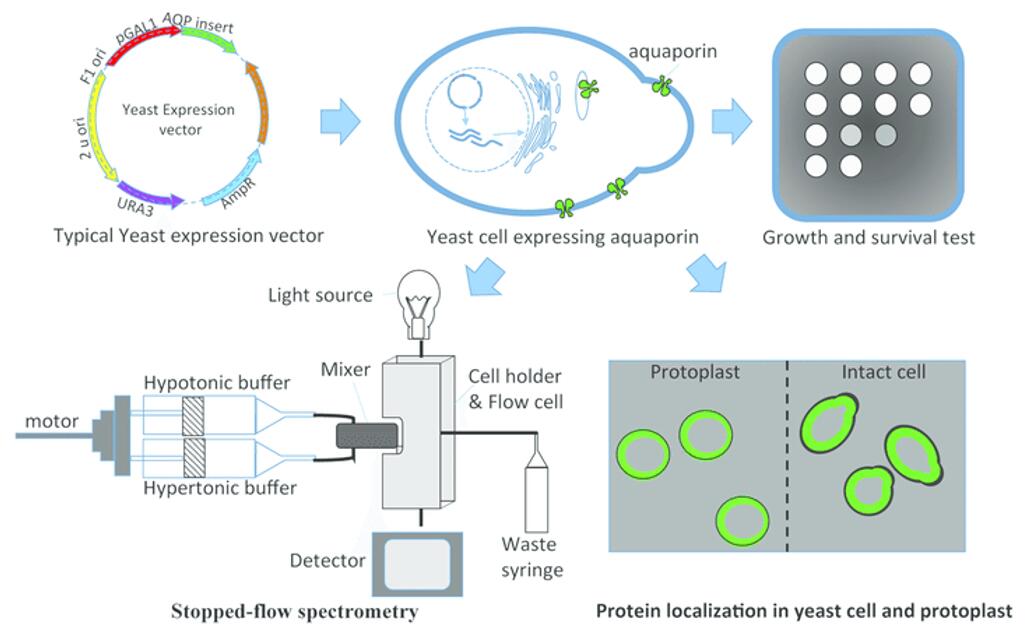 Fig.1 Yeast heterologous expression system used to evaluate aquaporins. (Deshmukh R, et al. 2016)
Fig.1 Yeast heterologous expression system used to evaluate aquaporins. (Deshmukh R, et al. 2016)
Among them, Pichia pastoris has the advantages of a fast growth rate, abundant commercial expression vectors and efficient secretory expression, and is the first choice for yeast expression of recombinant proteins.
Service Overview
Our company has extensive experience in yeast protein expression, having successfully expressed over 100 recombinant proteins. We offer services to screen high-copy yeast expression strains to meet the various specific needs of our customers.
Our yeast protein expression system services include, but are not limited to, the following. Customers can choose any of the above experiments, or a combination of related experiments, according to their requirements. Affinity purification, ionic column purification, hydrophobic packing purification, or molecular sieve purification are available.
- Construction of recombinant yeast expression plasmid (2 weeks).
- Electrotransformation of recombinant plasmid (1 week).
- Recombinant expression screening (3-4 weeks).
- Expression and purification of small-scale proteins (2-4 weeks).
- Expression and purification of large-scale proteins (Determine based on the expressed yield and volume).
In the yeast system expression process, the control of induction time and temperature, culture conditions and strain growth cycle directly affect the success or failure of recombinant protein expression. Our technicians are able to improve the success rate and expression level of expression by mastering and controlling the integration process.
We have successfully used yeast expression systems to express disulfide-bonded and glycosylated proteins, resulting in proteins with similar natural structures and post-translational modifications, ensuring protein activity.
Advantages of Our company Pichia Expression System
- The fermentation medium culture of Pichia pastoris is reasonably cost-effective and the products are easy to isolate. Generally, the carbon source is glycerol or glucose and methanol, and the rest are inorganic salts. The medium is protein-free, which facilitates the isolation and purification of downstream products.
- Foreign genes can be integrated into the Pichia pastoris genome at high copy numbers, which are not easily lost and can lead to highly expressed strains.
- As a eukaryotic expression system, Pichia pastoris has the subcellular structure of eukaryotes with post-translational modifications and processing, such as glycosylation, fatty acylation, protein phosphorylation, etc.
If you are looking for smarter, higher quality solutions that incorporate best practices, please feel free to contact us.
Reference
- Deshmukh R, et al. (2016). "Plant Aquaporins: Genome-Wide Identification, Transcriptomics, Proteomics, and Advanced Analytical Tools." Frontiers in Plant Science.7.
Related Services
It should be noted that our service is only used for research, not for clinical use.

 Fig.1 Yeast heterologous expression system used to evaluate aquaporins. (Deshmukh R, et al. 2016)
Fig.1 Yeast heterologous expression system used to evaluate aquaporins. (Deshmukh R, et al. 2016)