Every step in the drug Our company offers in vivo pharmacokinetic (PK) services for our customers' cutting-edge scientific studies because of its outstanding in vivo PK pharmacokinetic knowledge. All PK studies can be customized upon request. We offer full in-vivo PK support, including study planning and sample analysis, and can do so as a standalone service, as part of a comprehensive development program, or as a full-service single study package.
Service Overview
In vivo pharmacokinetics is a discipline that applies kinetic principles to study the absorption, distribution, metabolism, and excretion of drugs in the body. It aims to understand the patterns and forms of drug changes in the body and to assess the effects of drugs as part of drug research. In vivo PK studies are based on the qualitative and quantitative analysis of the content of drugs or their metabolites in biological samples.
Due to the complex matrix composition of biological samples, low concentration of analytes, high concentration variation, and a large number of samples to be tested, the establishment of efficient, accurate, and high-throughput methods for the analysis of biological samples has become crucial for pharmacokinetic studies. Our company can provide high-quality one-stop PK services for in vitro ADME, in vivo pharmacokinetics, and bioanalysis.
Research Capabilities
Our company has the ability to provide clients with in vivo pharmacokinetic data for drugs, toxicokinetic data, and the development and screening of solvent formulations in the drug development and preclinical phases. Test compounds include small molecule drugs, peptides, nucleosides, and nucleotides, as well as some biomolecules. Model animal species include different species of mice, rats, guinea pigs, rabbits, mini pigs, dogs, and monkeys, covering the preclinical development of drugs.
Our research capabilities include but are not limited to:
- Solvent Development - Development of solutions, suspensions, and emulsions involved in the drug screening phase.
- High-throughput screening protocols for single drug delivery and assay N-in-one.
- Tissue Distribution - Detection of drug distribution in major organs and various tissues.
- Metabolic Studies - Metabolite identification, qualitative or quantitative analysis.
- Drug Excretion - Analysis of drug excretion rates in urine, feces, and bile.
- Detection of Free Drugs in Live Animals - Application of microdialysis techniques.
- Special Routes of Drug Administration - Including intravenous drip, capsule administration in rats, intratracheal administration, and gastrointestinal localization of drug delivery.
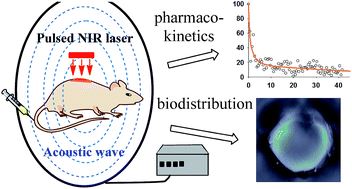 Fig.1 Real-time observation of star-shaped gold nanoparticles (AuNSs) in blood vessels, liver, spleen and kidney using multispectral photoacoustic tomography (MSOT). (Wang J, et al. 2015)
Fig.1 Real-time observation of star-shaped gold nanoparticles (AuNSs) in blood vessels, liver, spleen and kidney using multispectral photoacoustic tomography (MSOT). (Wang J, et al. 2015)
Overall Solutions
| Project Name | In Vivo Pharmacokinetic Service |
| Service Details | - Plasma kinetic studies
- Tissue distribution studies
- Excretion studies
- Material balance
- Blood-brain barrier
|
| Deliverables | Within agreed time, we will provide the summary to experiment data and conclusions, as well as a final experiment report. |
| Cycle | Decide according to your needs. |
Our Advantages
- Flexible study design with multiple drug delivery routes.
- Excellent service platform, professional technical team, advanced instruments, and equipment.
- High efficiency, fast response, flexible study direction, low price, short cycle time, and high quality.
- All experimental procedures are in accordance with international standards.
- Immunological methods are available for measuring drug concentrations, biomarkers, and/or neutralizing antibodies.
- Availability of high-resolution LSC and LC-MS quantification, HPLC, and UPLC analysis.
For more information, please feel free to contact us.
Reference
- Wang J, et al. (2015). "In vivo Pharmacokinetic Features and Biodistribution of Star and Rod Shaped Gold Nanoparticles by Multispectral Optoacoustic Tomography." RSC Adv. 5: 7529-7538.
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 Real-time observation of star-shaped gold nanoparticles (AuNSs) in blood vessels, liver, spleen and kidney using multispectral photoacoustic tomography (MSOT). (Wang J, et al. 2015)
Fig.1 Real-time observation of star-shaped gold nanoparticles (AuNSs) in blood vessels, liver, spleen and kidney using multispectral photoacoustic tomography (MSOT). (Wang J, et al. 2015)