The use of antibody-drug conjugates (ADCs) in oncology is crucial. Because there are so many different ADC molecular assemblages, each ADC development program has unique difficulties. ADC analysis necessitates sophisticated bioanalytical techniques to guarantee reliable reporting of all pharmacokinetic and immunogenicity data.
Our company offers a complete ADC preclinical development platform to streamline the challenging nature of ADC analysis and deliver fast, trustworthy data. Through years of practical experience, careful preparation, rigorous execution, and accurate outcomes, we provide for our customers.
Our ADC R&D Service Platform
ADC drugs consist of three components: antibodies, couplers (connectors) and payloads. Our company's ADC development platform has supported several ADC studies in the pharmaceutical and biopharmaceutical sectors, which include the provision of toxin small molecules (DM1, MMAE, Dxd, Exatecan, SN38, etc.), the provision of targets (Her2, Her3, Claudin18.2, Trop2, CD33, Muc1, FR, etc.).
Also, we have extensive expertise in creating and validating analytical methods for different targets, and we can efficiently assess the expression level and accessibility of targets as necessary to offer helpful target selection recommendations.
The following services are available from our ADC R&D service platform:
Synthesis of ADC Payloads
Our company offers a wide range of chemical ADC payloads with different mechanisms of action for customers to choose from. We also offer a custom synthesis of payloads based on customer requirements.
- Microtubule protein inhibitors.
- DNA damage agents.
- Immunomodulators.
- Self-developed payloads.
- We provide payloads and derivatives of commercially available ADCs.
ADC In Vitro Assays
With hundreds of cancer cell lines, Our company offers a wide selection of ADC target protein-positive and negative tumor cells. In addition, we have extensive experience in cell labeling and FACS-based cell viability analysis to help screen for optimal antibodies. Our available services include ADC binding assays and ADC internalization (confocal imaging).
Pharmacological Studies of ADCs
Our company's research services include target antigen binding activity, target antigen-related pharmacology, and mechanism of action of payloads and metabolites (with a focus on the differences in pharmacological mechanisms of ADCs, naked antibodies, payloads, and metabolites).
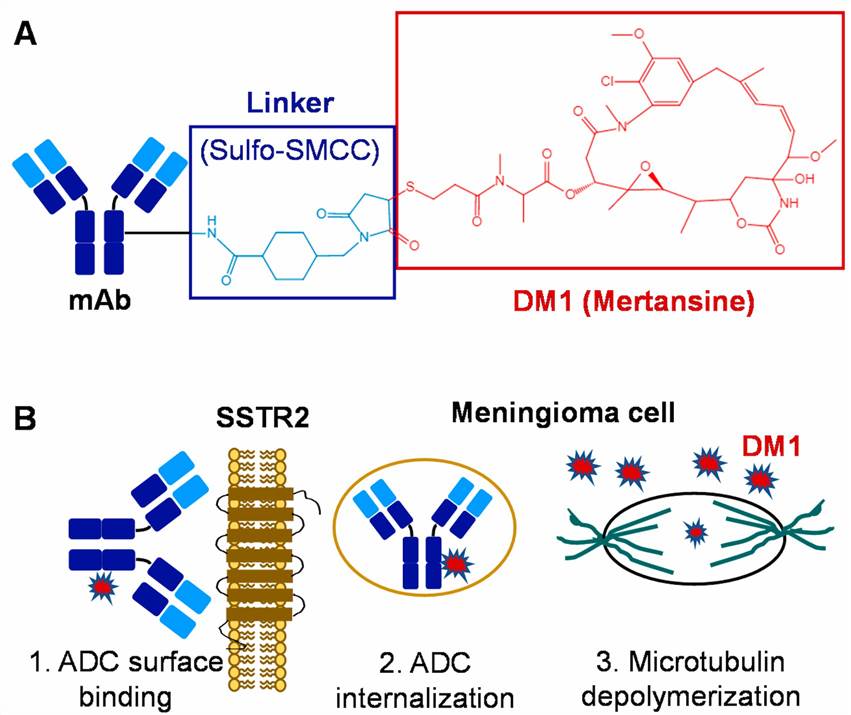 Fig.1 ADC-based targeted therapy for meningiomas. (A) Construction and structure of ADC. (B) Mechanism of ADC to treat meningiomas. (Chen K, et al. 2021)
Fig.1 ADC-based targeted therapy for meningiomas. (A) Construction and structure of ADC. (B) Mechanism of ADC to treat meningiomas. (Chen K, et al. 2021)
Pharmacological evaluation of ADCs
Our company has hundreds of tumor models for ADC efficacy evaluation.
- Tumor models for multiple oncological diseases
- Multiple model options (xenograft models, homozygous models, in situ xenograft models, transgenic models, hPBMC/CD34+ HSC humanized models)
ADC Pharmacokinetic Studies
Our company provides high-quality quantitative analysis for key parameters in ADC pharmacokinetic studies, presenting accurate results.
| Analyte | Description | Analysis Methods |
| Conjugated Antibody | An antibody with a minimum of DAR≧1 | Ligand binding assays (LBAs) |
| Total Antibody | Conjugated, partially unconjugated, and fully unconjugated (DAR≧0) | LBAs |
| Small Molecules | Released/free small molecules and their metabolites | Liquid Chromatography with tandem mass spectrometry (LC-MS-MS) |
| Adenosine deaminase (ADA) | Antibodies against antibodies of ADC, linker or drug | LBAs |
ADC Immunogenicity
An important consideration when assessing biological therapy is immunogenicity. It may lessen the effectiveness of ADCs and raise the likelihood of adverse effects. Our company fully understands the complexities of ADA evaluation and provides comprehensive immunogenicity testing for our customers.
ADC Safety Assessment
Our safety assessment services include:
- Single dose/repeat dose toxicity.
- Tissue cross-reactivity.
- ADA testing.
For more information, please feel free to contact us.
Reference
- Chen K, et al. (2021). "Antibody-Drug Conjugate to Treat Meningiomas." Pharmaceuticals. 14(5): 427.
Related Services
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 ADC-based targeted therapy for meningiomas. (A) Construction and structure of ADC. (B) Mechanism of ADC to treat meningiomas. (Chen K, et al. 2021)
Fig.1 ADC-based targeted therapy for meningiomas. (A) Construction and structure of ADC. (B) Mechanism of ADC to treat meningiomas. (Chen K, et al. 2021)