Subchronic and chronic toxicity studies provide information on possible health hazards that may arise from repeated exposure over a significant portion of the life span of the test species. Studies offer information on the toxicity of chemicals that show the potential for accumulating in target organs. Also, they can offer estimates of levels of unobserved deleterious effects to aid in developing safety standards for human exposure.
Our company is aware that investigations into subchronic and chronic toxicity can offer insight into the long-term effects of test compounds on both animals and people. We offer specialized subchronic/chronic toxicity study services to our clients in order to look into the impacts of test articles on homeostasis, bodily function, illness induction, and lifespan. To better support your clinical studies, we constantly strive to deliver the most thorough and legal chronic and subchronic toxicity research results.
Service Overview

Our company can conduct long-term safety testing on your medicinal entity, as required for your preclinical drug development program if it is intended for repeated or long-term usage in patients.
Our subchronic toxicity studies range in length from three to twelve months. Depending on the species being studied, testing for chronic toxicity studies can last anywhere between 26 and 52 weeks. Subchronic and chronic toxicity studies are typically conducted on one rodent and one non-rodent, but NHPs are also used.
Oral, cutaneous, and inhalation are the three main delivery methods utilized in subchronic and chronic toxicity investigations. The test substance's physical and chemical characteristics as well as the principal human exposure route influence the route selected.
The goal of chronic and subchronic toxicity studies is to characterize dose-response relationships, identify target organs, test additional hypotheses about the mode of action, predict the health effects of your therapeutic entity in human exposures and establish dose levels that won't adversely affect long-term or subchronic use.
Research Capabilities
For rodents, we should normally use at least 20 animals per group per sex. And for non-rodents, at least 4 animals per group per sex should be used. A concurrent control group should be used along with a minimum of three dose levels.
The frequency of exposure is typically once per day, but may differ depending on the chosen route (oral, cutaneous, or inhalation) and should be modified for the test substance's toxicokinetic profile. In addition to measurements (such as weight) and periodic in-depth observations (such as hematological tests, urine, and clinical chemistry), the study should also involve autopsies and histological analyses.
- Subchronic Toxicity Studies
The trial has one carrier control group (40 animals) at 3 dose levels per sex per group of 25 animals. For the whole study, the control group should receive the same dosage of 0.9% physiological saline. Daily clinical observations, weekly body weight, and feed consumption data are all study measurements.
At the conclusion of the dosing phase, twenty animals were necropsied, and those that survived were put into a recovery period. Any animals that passed away or were executed had necropsies performed on them, and the weights of the major organs were noted. Examinations of ophthalmology, hematology, clinical chemistry, and urinalysis were among the clinical observations.
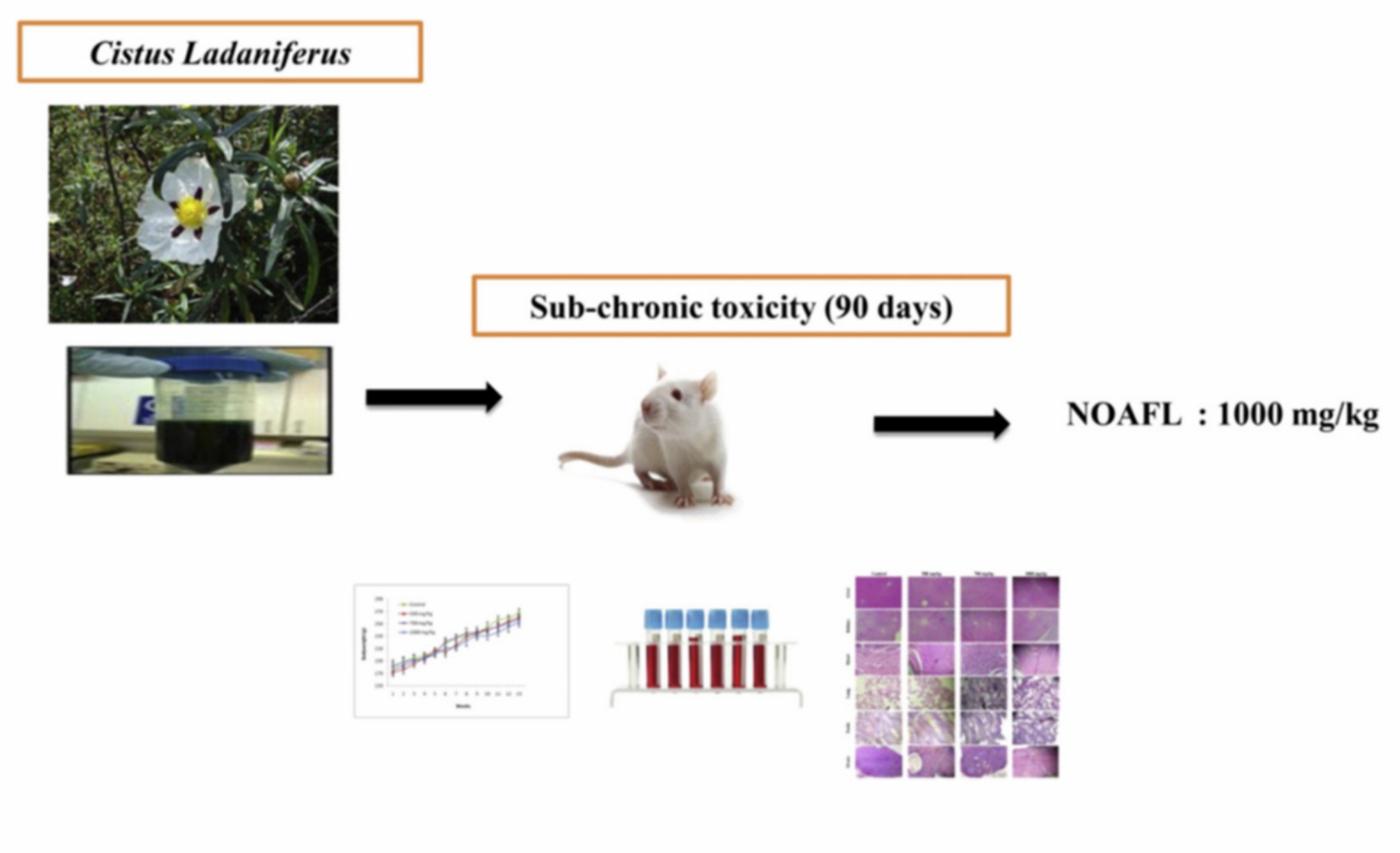 Fig.1 Sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. (Kabbaoui M, et al. 2017)
Fig.1 Sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. (Kabbaoui M, et al. 2017)
Overall Solutions
| Project Name | Subchronic and Chronic Toxicity Study Service |
| Study Objectives | - Establish a "no observed effect level" (NOEL)
- Characterize the dose-response relationship after repeated dosing
- Identify and characterize the specific organs affected after repeated dosing
- Predict a reasonable and appropriate dose (maximum tolerated dose or MTD) for chronic exposure studies
|
| Deliverables | Within agreed time, we will provide the summary to experiment data and conclusions, as well as a final experiment report. |
| Cycle | Decide according to your needs. |
For more information, please feel free to contact us.
Reference
- Kabbaoui M, et al. (2017). "Acute and Sub-Chronic Toxicity Studies of The Aqueous Extract from Leaves of Cistus Ladaniferus L. in Mice and Rats." Journal of Ethnopharmacology. 209: 147-156.
Related Services
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 Sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. (Kabbaoui M, et al. 2017)
Fig.1 Sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats. (Kabbaoui M, et al. 2017)