Studies on the maximum tolerated dosage (MTD) identify the highest dose of a medication or treatment that won't result in unfavorable side effects or toxicity. These investigations aid in establishing secure starting doses for human clinical trials and could continue throughout the creation of clinically viable treatments. Our company offers our customers professional MTD study services.
Preliminary in vivo toxicity studies are routinely carried out by our team of toxicologists to establish the ideal dose, frequency, and route of administration. For both single-dose and multiple-dose administration of novel substances, our team has the expertise and knowledge to assist you in determining the maximum tolerated dose.
Service Overview
The studies conducted by Our company are created to quickly and accurately assess the toxicological and toxicokinetic quality of potential new chemical entities in the drug development process for our customers, facilitating later regulatory toxicology studies and potentially allowing for an earlier start to clinical trials.
In order to specify doses that may be employed in upcoming trials, we provide customizable study designs. Compounds are initially assessed for tolerability in a single dose escalation phase, when the test system is given a single dose that is anticipated to produce an effective dose-related multiple. Data from this phase is used to determine the dose for the repeat dose phase as well as to estimate the maximum tolerated dose for a single dose administration. Studies using various designs, whether single or repeated doses, may reveal symptoms of toxicities that are dose-limiting and enable the choice of dose levels for regulatory investigations.
Research Capabilities
Acute toxicity studies, short-term dose escalation studies, and dose-ranging studies can all be used to calculate MTD. These investigations are planned with a minimal number of animals and contain clinicopathology endpoints like blood tests for liver function and toxicological endpoints like clinical observations. The evaluation of long-term safety then uses this maximum tolerated dose.
Our company commonly uses many animal models for MTD research. The greatest dose of a substance that may be delivered without experiencing negative effects or substantial toxicity is determined in these short-term experiments (often lasting 7 days). Prior to in vivo efficacy testing, MTD studies are frequently carried out to help with dose selection.
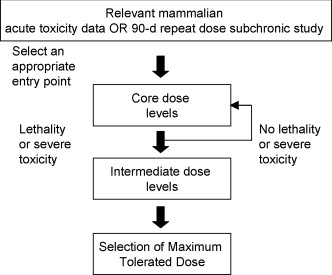 Fig.1 General principle of mammalian toxicology MTD procedure. (Hutchinson T. H, et al. 2009)
Fig.1 General principle of mammalian toxicology MTD procedure. (Hutchinson T. H, et al. 2009)
The need for and design of an MTD study depends on what is known about the test compound and its pharmacokinetics. We administer test compounds to animals at a range of doses and at one or more dosing frequencies. The animals are also observed for side effects. During treatment, we also take blood samples, and at the conclusion of the research, we typically take tissue samples for analysis.
Overall Solutions
| Project Name | Maximum Tolerated Dose Study Service |
| Service Details | Mice, rats, rabbits, canines, felines, hamsters, mini pigs, NHP Dermal, dietary, intramuscular, intraperitoneal, gavage, infusion, intravenous, subcutaneous |
| Deliverables | Within agreed time, we will provide the summary to experiment data and conclusions, as well as a final experiment report. |
| Cycle | Decide according to your needs. |
For more information, please feel free to contact us.
Reference
- Hutchinson T. H, et al. (2009). "Benefits of The Maximum Tolerated Dose (MTD) and Maximum Tolerated Concentration (MTC) Concept in Aquatic Toxicology." Aquatic Toxicology. 91(3): 197-202.
Related Services
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 General principle of mammalian toxicology MTD procedure. (Hutchinson T. H, et al. 2009)
Fig.1 General principle of mammalian toxicology MTD procedure. (Hutchinson T. H, et al. 2009)