Acute toxicology studies focus on the toxicological effects following a single large dose of a substance of interest. Our company offers our customers skilled services for acute toxicological studies. Our simplified, well-designed acute toxicity studies can aid in the choice of dosages for preliminary repeat-dose toxicity testing as well as in the prediction of potential target organ harm.
Our company is dedicated to offering effective preclinical drug development study designs that ensure proper scientific rigor is attained. This will enable the evaluation of acute systemic toxicity, skin and eye irritation, and skin sensitization.
Service Overview

Acute toxicity tests by Our company are intended to assess adverse effects that happen right away or quickly (within 24 hours) following a single or several doses of a chemical. Any response that results in organ malfunction and/or biochemical lesions that may change the function of the entire organism or specific organs is considered an unfavorable reaction. Hence, to fully examine the short-term effects of our customers' test programs, our study designs often concentrate on assessing these endpoints. Many in vivo techniques that have been approved by regulatory bodies are used by us to carry out experimental investigations into acute toxicity.
Research Capabilities
Our company offers a wealth of knowledge in acute toxicity under GLP, including the biocompatibility testing necessary for medical devices. To meet the regulatory requirements for acute toxicity testing, we combine in vivo acute toxicology testing with in vitro toxicology studies.
The test portfolio offers details on the potential health risks connected to transient exposure to the test substance via various administration routes. Each test is intended to carry out a limit test or to calculate the product's concentration.
| Inhalation Toxicity | - Acute inhalation toxicity, OECD 403
- Acute inhalation toxicity: fixed concentration procedure, OECD 433
- Acute inhalation toxicity: acute toxic class method, OECD 436
|
| Oral Toxicity | - Acute oral toxicity: fixed-dose procedure, OECD 420
- Acute oral toxicity: up-and-down procedure, OECD 425
- Acute oral toxicity: acute toxic class method, OECD 423
|
| Skin Sensitization | - Skin sensitization - local lymph node assay, OECD 429
- In vitro skin sensitization: ARE-Nrf2 luciferase test method, OECD 442D
- Skin sensitization: local lymph node assay: BrdU-ELISA or -FCM, OECD 442B
|
| Skin Irritation/Corrosion | - Acute dermal irritation/corrosion, OECD 404
- In vitro skin corrosion: human skin model test, OECD 431
- In vitro skin irritation: reconstructed human epidermis test method, OECD 439
|
| Dermal Toxicity | - Acute dermal toxicity, OECD 402
|
| Eye iIrritation/Corrosion | - Acute eye irritation/corrosion, OECD 405
- Bovine corneal opacity and permeability test method for identifying, OECD 437
- Short time exposure in vitro test method for identifying, OECD 491
|
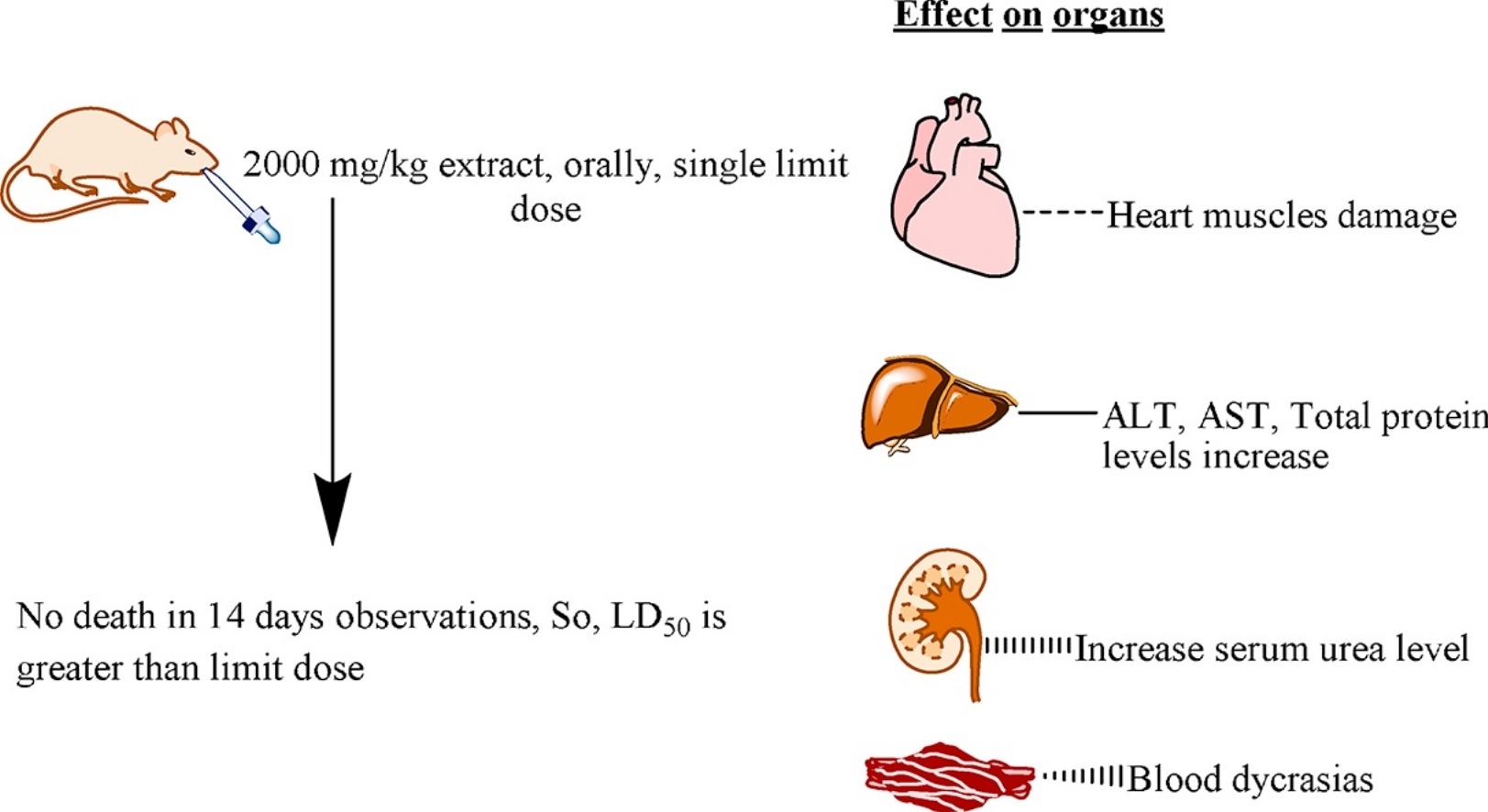 Fig.1 Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. (Saleem U, et al. 2017)
Fig.1 Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. (Saleem U, et al. 2017)
Overall Solutions
| Project Name | Acute Toxicology Study Service |
| Service Details | - Acute toxicology study design and development
- Skin irritation and corrosion testing
- Eye irritation testing
- Skin sensitization testing
- Photosensitization/phototoxicity testing
- Lethality testing
- Acute systemic toxicity testing
- Acute inhalation testing
- Acute toxicology statistical analysis
- Safety considerations for administration via the parenteral route
|
| Deliverables | Within agreed time, we will provide the summary to experiment data and conclusions, as well as a final experiment report. |
| Cycle | Decide according to your needs. |
For more information, please feel free to contact us.
Reference
- Saleem U, et al. (2017). "Acute Oral Toxicity Evaluation of Aqueous Ethanolic Extract of Saccharum Munja Roxb. Roots in Albino Mice As per OECD 425 TG." Toxicology Reports. 4: 580-585.
Related Services
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. (Saleem U, et al. 2017)
Fig.1 Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. (Saleem U, et al. 2017)