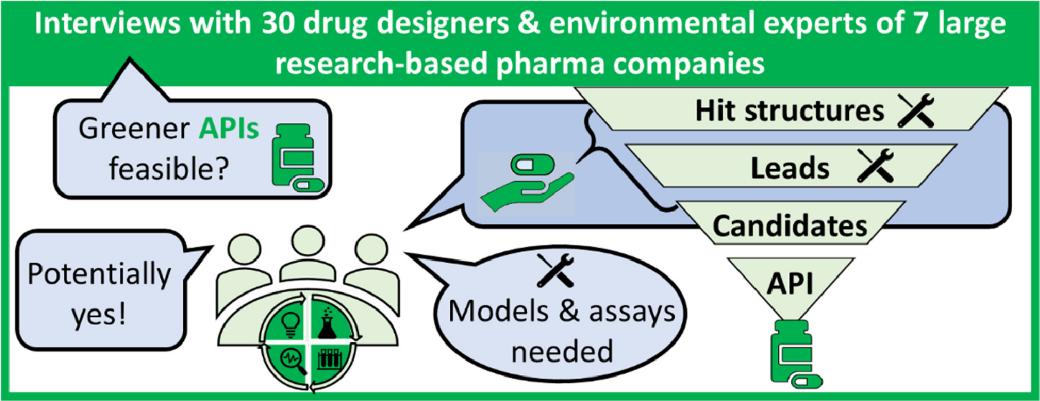
The human and environmental health implications of both APIs and their transformation products (TPs) alongside their metabolites demand attention. Recently, a structured questionnaire enabled Neele Puhlmann et al. to interview 30 experts from seven global pharmaceutical companies about the industry's needs and opportunities for creating greener APIs. [2]
Drug development can successfully integrate environmental factors throughout various R&D stages including both the initial screening phase and the subsequent lead compound optimization stage. Pharmaceutical R&D's inherent flexibility allows this integration because its processes require balancing many different factors. Research and development must include environmental factors from the beginning of the process especially around molecule screening and optimization stages. It may also bring new business opportunities. The latest opportunity is in the later stages of lead compound optimization, because the molecular structure of the API candidate will no longer change thereafter.
Additionally, the availability of effective in silico models and in vitro assays is vital for incorporating these environmental considerations, as their characteristics, such as throughput and cost, dictate their applicability at different stages of the process.




 (Neele Puhlmann, et al. 2024)
(Neele Puhlmann, et al. 2024)