Peptide-drug conjugates (PDCs) are emerging as a promising class of therapeutics following antibody-drug conjugates. It consists of a cytotoxic payload, a homing peptide, and a linker connecting the peptide and the cytotoxic payload that combines the targeting capabilities of peptides with the pharmacological properties of small molecule drugs [1]. By linking peptides to drug molecules, PDCs can enhance drug delivery, improve efficacy, and reduce off-target effects. This innovative approach has the potential to revolutionize the treatment of various diseases, including cancer, infectious diseases, and autoimmune disorders. Despite their significant potential, obstacles such as poor stability, suboptimal targeting, and potential payload toxicity can impede the advancement of PDCs through preclinical and clinical development.
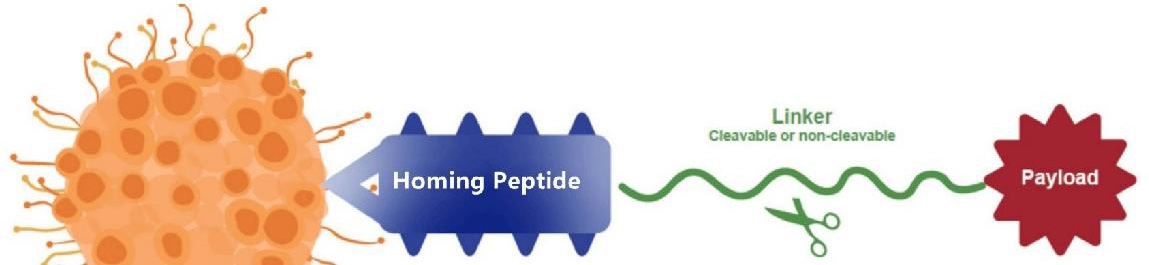 Fig 1. Structure of PDCs.
Fig 1. Structure of PDCs.
At our state-of-the-art PDCs R&D platform, we offer a comprehensive range of services to support the development of peptide-drug conjugates. Our expertise in peptide synthesis and drug development enables us to deliver customized solutions to meet PDCs development process of our clients.
Our Peptide-drug Conjugate R&D Platform
Our platform offers a wide range of services to support the design, synthesis, analysis, and development of peptide-drug conjugates. The following services are available from our PDC R&D service platform:
1. Computational Modeling for PDCs Design
Our team of computational scientists leverages advanced modeling techniques to predict the binding affinity and pharmacokinetic properties of PDCs. By simulating the interactions between targeting peptides, linkers, and payloads, we can optimize the design of PDCs for enhanced target specificity and drug release kinetics.
2. Design and Synthesis of PDCs
We specialize in the design and synthesis of peptide-drug conjugates tailored to specific therapeutic targets. Our capabilities include:
- Design and synthesis of diverse cancer-targeting peptides.
- Create linkers that are the ideal size, have an appropriate release mechanism, and work well with the desired conjugation chemistry.
- Develop payload derivatives that have reduced toxicity or are linked to specific sites.
- Prepare personalized drug-linker combinations.
Our platform encompasses a wide range of sample payloads, including MMAE, DM1, DM4, PBD, duocarmycins (DC), Doxorubicin derivatives, Camptothecins and more, each carefully selected for their unique properties and therapeutic potential. Moreover, our platform offers a comprehensive selection of sample linkers, such as MC-VC-PAB-PNP, Glu-VC-PAB, NHS-Glu-VC-PAB, Azido-Glu-VC-PAB, Hydrazone (acid sensitive), Disulfide (Glutathione sensitive), SMCC, among others.
3. PDCs Analysis
To assess the efficacy and safety of PDCs, we offer a comprehensive range of analytical services, including but not limited to:
- Structural characterization of PDCs
- Evaluating the targeting specificity of PDCs towards their intended molecular
- In vitro assays to evaluate the binding affinity and biological activity of the peptide and drug components
- In vitro and in vivo efficacy studies in cell culture and animal models
- Stability testing, pharmacological studies and safety assessment
4. DMPK Services for PDCs
Our expertise in drug metabolism and pharmacokinetics (DMPK) allows us to evaluate the absorption, distribution, metabolism, and excretion properties of PDCs. The services include:
- In vitro ADME studies: It involves investigating the absorption, distribution, metabolism, and excretion properties of the drug conjugate outside of the body. This includes assessing the stability of the PDC in different biological fluids, determining its binding affinity to plasma proteins, assessing its potential for drug-drug interactions, and evaluating its metabolic stability in various liver microsomal or hepatocyte systems.
- In vivo PK studies: It involves evaluating the pharmacokinetic properties of the drug conjugates inside the body. This includes assessing the absorption, distribution, metabolism, and excretion of the PDC in animal models (such as rodent models)or human clinical trials.
5. PDCs Preparation R&D
At our company, we offer extensive formulation development services for PDCs to help improve the stability, efficacy, and bioavailability of these complex molecules. Our experienced team of scientists and researchers can:
- Develop long-term new preparation such as slow-release microspheres, slow-release gels, nano-preparations, etc.
- Optimize your PDCs formulations to improve their stability, solubility, and bioavailability.
- Conduct comprehensive preformulation studies to identify the most suitable formulation strategies for your PDCs.
- Develop and validate analytical methods for your PDCs in various formulations.
- Conduct stability testing studies to evaluate the shelf-life and degradation pathways of your PDCs.
- Scale-up and tech transfer.
6. PDCs Generic Drug Development
In addition to custom PDCs design services, we offer generic drug development solutions to expedite the development of novel PDCs formulations. Our expertise in formulation optimization, process validation, and regulatory compliance enables us to accelerate the commercialization of PDC products.
For more information, please feel free to contact us.
Reference
- Lindberg J., et al. Progress and future directions with peptide-drug conjugates for targeted cancer therapy[J]. Molecules, 2021, 26(19): 6042.
Related Services
It should be noted that our service is only used for research, not for clinical use.

 Fig 1. Structure of PDCs.
Fig 1. Structure of PDCs.