Approval of a veterinary drug requires, at a minimum, proof that the veterinary drug is effective and safe. Our company has many years of experience in many different types of target animal safety studies and can perform ADME, pharmacokinetic, bioequivalence, residue removal and targeted animal safety (TAS) studies in accordance with international regulations.
We provide professional veterinary TAS testing services using clinical data (physical examination, food consumption, body weight, etc.), clinical pathology data, gross necropsy results, organ weights, and histopathology results in targeted animal safety studies to determine the potential toxicity associated with the administration of veterinary products and to confirm adequate margins of safety for the target species.
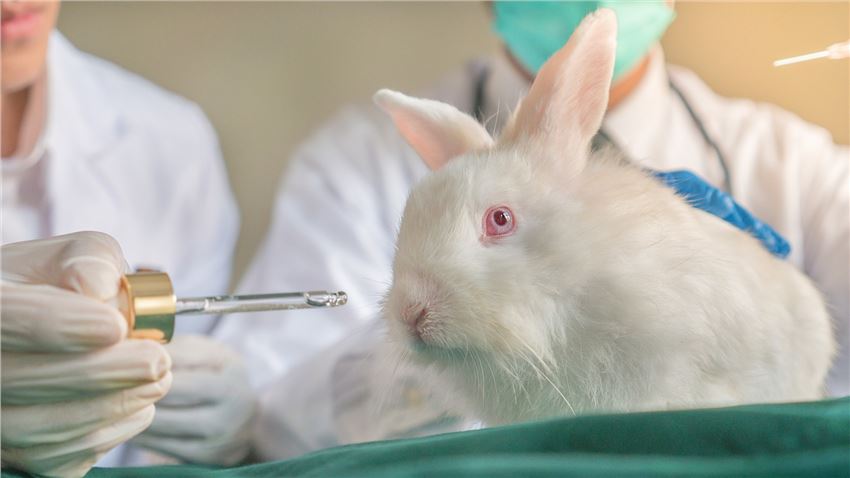
Learn About Our Veterinary TAS Test Service
Our company's TAS evaluations are suitable for determining the safety of investigational veterinary drug products in target animals, including identification of target organs (where possible) and confirmation of safety factors, using the minimum number of animals suitable for the study.
Our design of the TAS evaluation and prediction of potential adverse reactions that may occur in the target species will be informed by data including published literature and preliminary studies, including pharmacokinetics, pharmacodynamics, and toxicology of target and non-target experimental animal studies.
We perform appropriate observations, physical examinations, clinicopathological examinations (hematology, blood chemistry, urinalysis, fecal analysis, etc.), necropsies, and histopathological examinations to determine possible adverse effects of veterinary products.
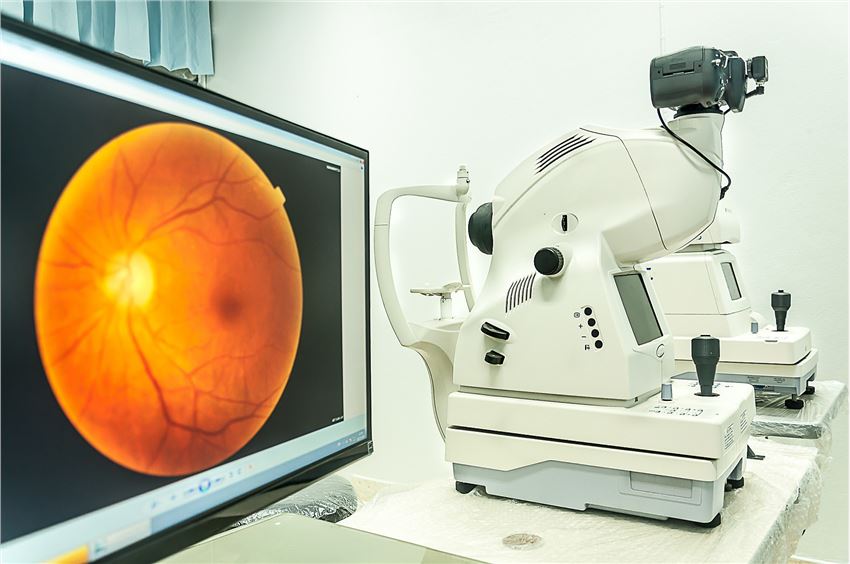
Clinicopathological testing of target animal species.
| Hematology | Coagulation | Clinical chemistry | Urinalysis | Acute phase markers |
| General recommendations | RBC, HGB, HCT, MCV, MCH, MCHC, RDW, reticulocyte count, platelet count, total and differential leukocyte (WBC) counts, etc. | PT, APTT, fibrinogen | ALT, AST, ALP, GGT, CK, total bilirubin, urea, creatinine, glucose, total cholesterol, triglycerides, total protein, albumin, globulins, etc. | Color, clarity, volume, specific gravity, pH, protein, etc. | SAA appropriate for all species |
| Species-specific recommendations |
| Dog and cat | | | | | CRP (dog), AGP (cat) |
| Pigs | | | SDH and/or GDH as a replacement for ALT | | Haptoglobin, Pig-MAP |
| Horses, cattle, sheep, goats | Reticulocyte count optional for horses | | SDH and/or GDH as a replacement for ALT Magnesium | | Haptoglobin |
Tolerance testing of target animal species.
Our company's experts can design tolerance studies in target animal species to meet the specific requirements of regulatory submissions. The flexibility of the protocol allows us to pay particular attention to the actual use of the product by carefully selecting dose levels and animal population sizes.
- Target animal species: Cattle, sheep, goats, pigs (including neonates), poultry, marine fish, and freshwater fish.
- Route of administration: Topical and dermal, oral, intravenous, intraperitoneal, intramuscular, subcutaneous.
The types of new veterinary drugs we can research are as follows:
- Biological products, including vaccines, serum products, diagnostic products, microecological products, etc.
- Other veterinary drugs, including chemicals, antibiotics, disinfectants, biochemical drugs, radiopharmaceuticals, and topical insecticides.
- Chinese medicine preparations, including Chinese herbal medicines, and Chinese patent medicines.
For more information, please feel free to contact us.
Related Solutions
It should be noted that our service is only used for research, not for clinical use.


