The sensitization test is a test to determine whether the subject is an allergen and the strength of allergenicity. Our company provides professional skin sensitization testing services for a wide range of products such as chemicals, cosmetics, and skincare products according to appropriate testing standards, as well as non-standard testing services based on customer requirements.
Principle of Sensitization Test
The sensitizing chemical enters the body through a certain pathway, binds with tissue proteins to form antigens, and stimulates immunologically active cells to produce sensitizing lymphocytes or humoral antibodies.
After a sensitization period of 1-2 weeks, the immune response is fully developed and a certain number of sensitized lymphocytes or specific antibodies are formed.
When the re-exposure causes the body to produce a state of heightened sensitivity to the chemical, which manifests itself in a certain abnormal form.
Test Purpose
- To assess the toxicity of the test substance and provide a basis for grading the degree of skin sensitization in mammals.
- Determine whether repeated exposure to the test substance can cause dermal sensitization in mammals.
- Provide a basis for the selection of doses for other toxicological tests.
- Facilitate labeling management.
Test Items
Our company offers a wide range of skin sensitization tests, including but not limited to the following:
- Single-use hygiene product skin sensitization test.
- Skin sensitization tests for cosmetic products.
- Chemical skin sensitization test.
- Contact skin sensitization tests.
- Skin irritation sensitization test.
- Skin sensitization test for antimicrobial textiles.
- Medical device irritation and delayed hypersensitivity test.
- Medical device irritation and delayed hypersensitivity test (two infusion solutions).
About Skin Sensitization Test Service
Test Animals
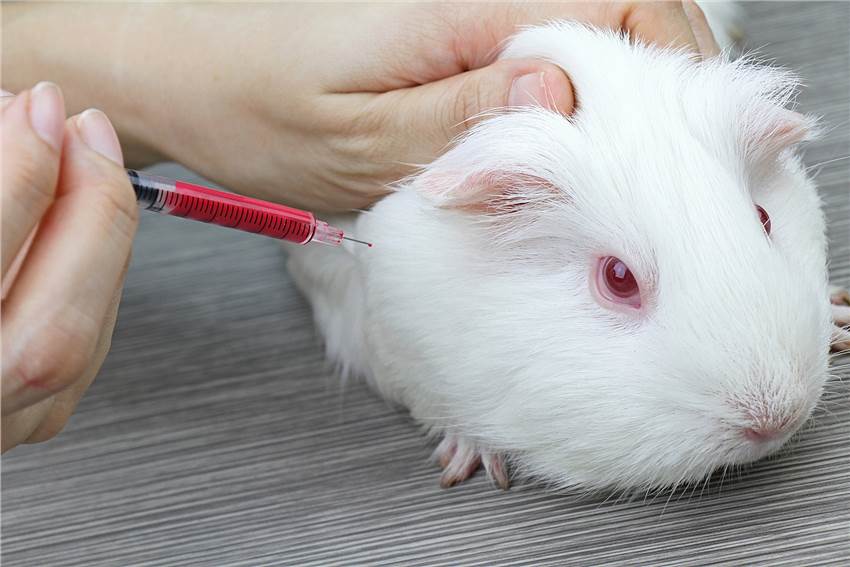
Our company uses healthy, adult female or male guinea pigs and mice.
Test Methods
Our company currently offers three animal tests to determine the potential skin sensitization of test samples, including two guinea pig tests and one mouse test.
- Local Lymph Node Assay (LLNA)
The LLNA is administered to experimental animals through the ear and causes lymphocyte proliferation in the lymph nodes in proportion to the dose of the test substance. Using a radiolabeling method, the labeling rate of lymphocytes in the test and control groups is measured and compared to evaluate the sensitization intensity.
We measured the proliferation of lymphocytes in the lymph nodes draining from the application site of the mouse ear after the test sample was disposed of locally on the back of the ear.
- Skin Sensitization Test for Guinea Pigs
The Buchler test (BT) and guinea pig maximization test (GPMT) are performed on guinea pigs after repeated dermal application (induction exposure) or intradermal injection of the test substance for 10 to 14 days (induction phase), administration of an excitation dose of the test substance, observation of the guinea pigs, and comparison of the intensity of the dermal response to the excitation exposure of the test substance with that of the control group. GPMT was used for single chemicals.
Skin Sensitization Test Report
The final test report we provide includes the following.
- Sample acceptance date and sealing of samples.
- Test start and end dates, test x project leader.
- Test Summary.
- Name of the test substance, active ingredient, purity, dosage form, physical and chemical properties, solvent and method used for preparation, etc.
- Experimental animal species, strain, grade, number, weight, sex, source, quarantine, adaptability. Laboratory animal feeding environment, including temperature, relative humidity, edible materials, and laboratory animal facilities use permit number.
- Dose and group, including the principles and basis for selecting the dose, the way animals are grouped, and the number of animals of each sex in each group.
- Test conditions and methods, including the main instruments and equipment, the route of contamination, the contamination protocol, the test period, the observation indexes, etc.
- Test results: Summarized in textual descriptions and tables, reporting the skin reaction and sensitization rate of each group of animals, etc. Mouse local lymph node analysis test reports the mean or individual DPM values and irritation indices for each dose group.
- Credibility checks, including the results of the most recent credibility test.
- Test conclusion: Give a conclusion as to whether the subject caused a sensitization reaction under the test conditions.
- Description of the original record keeping.
If you are looking for the best solution in the field of toxicology research, please feel free to contact us.
Related Solutions
It should be noted that our service is only used for research, not for clinical use.


