Our company has extensive experience in toxicology testing and offers an in vivo mammalian erythrocyte micronucleus test to determine if a test sample causes damage to erythrocyte chromosomes or mitotic apparatus that induces an increased incidence of micronuclei and to evaluate the mutagenic potential of the sample. Any chemical that causes chromosome breakage or damage to chromosome and spindle junctions can be detected by our micronucleus test.
Test Range
Veterinary drugs, pesticides, chemicals, fungicides, organic toxicants, solid waste, domestic waste leachate, hazardous waste, sewage, sludge, water quality, surfactants, etc.
About In vivo Mammalian Erythrocyte Micronucleus Test Service
The following are available from Our company's in vivo mammalian erythrocyte micronucleus testing service.
Test Cells
Multi-stained erythrocytes are a stage in the development of late-dividing erythrocytes from juvenile to mature erythrocytes. The sufficient number of multi-staining erythroblasts in bone marrow, the easy identification of micronuclei, and the low spontaneous rate of micronuclei make the bone marrow multi-staining erythroblasts the cell population of choice for the micronucleus test.
Basic Principles and Methods of The Test
We exposed the animals to the test samples by appropriate routes, executed the animals after a certain period of time, removed the bone marrow, prepared smears, fixed, stained, and counted the multi-stained erythrophils containing micronuclei under a microscope.
Designing the dose group → Animal staining → Taking the bone marrow cavity for staining → Microscopic observation → Counting of micronuclei multi-staining erythrophils → Evaluation of results
Test Animals
We routinely select adult healthy mice or rats.
Sample Processing
Usually, distilled water, isotonic saline, vegetable oil, edible starch, sodium carboxymethyl cellulose, etc. are used.
Route of Contamination
We usually use oral or intraperitoneal injection, but we can also use other reasonable routes according to customer requirements.
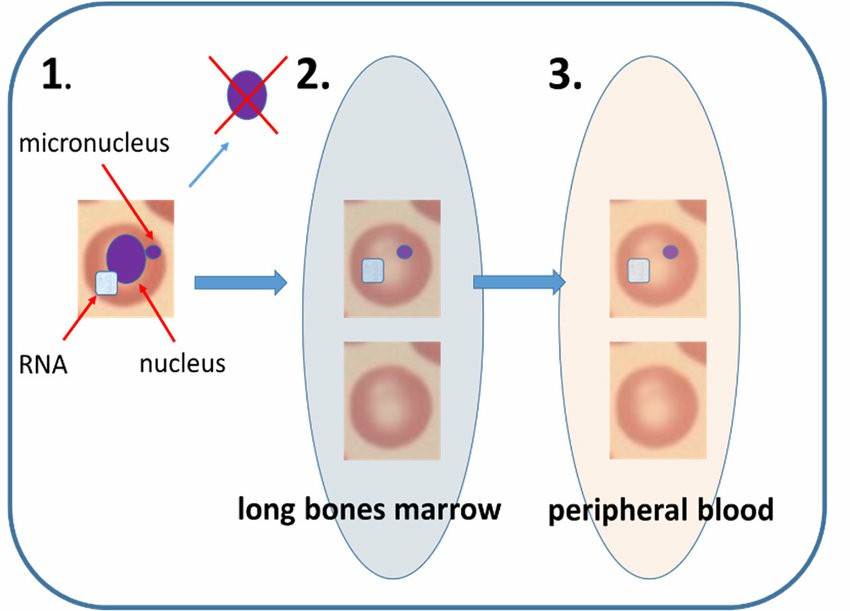 Fig.1 Mammalian erythrocyte micronucleus assay. (Sommer S, et al. 2020)
Fig.1 Mammalian erythrocyte micronucleus assay. (Sommer S, et al. 2020)
In vivo Mammalian Erythrocyte Micronucleus Test Report
The final test report Our company provides includes the following.
- Test name, test start and end dates.
- Summary of the test.
- Test sample: Physical and chemical properties, preparation method, the solvent used, and its solubility and stability to the sample.
- Animals: Breed, strain, weight, number, sex, source, feeding conditions, feed, etc.
- Laboratory animal feeding environment: Feed source, room temperature, relative humidity, laboratory animal room qualification number.
- Test methods: Main instruments and equipment, the basis of selected dose, dose conversion, route and mode of poisoning, test processing and production process.
- Test results: Poisoning performance, the mean and standard deviation of erythrocytes with micronuclei in each group, the proportion of erythrocytes in total erythrocytes, dose-response relationship, statistical analysis.
- Conclusion of the test: Give the conclusion of whether the test sample has a mutagenic effect under the test conditions.
- Description of the original record keeping.
For more information, please feel free to contact us.
Reference
- Sommer S, et al. (2020). "Micronucleus Assay: The State of Art, and Future Directions." International Journal of Molecular Sciences. 21(4): 1534.
Related Solutions
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 Mammalian erythrocyte micronucleus assay. (Sommer S, et al. 2020)
Fig.1 Mammalian erythrocyte micronucleus assay. (Sommer S, et al. 2020)