Our company has many years of experience in evaluating skin phototoxicity and can develop in vivo and in vitro phototoxicity evaluation methods according to international standards for the pharmaceutical, chemical and agrochemical industries. Study design to meet the standards and professional requirements of any test material evaluation.
Photobiological Studies
Our scientists can create tailored exposure settings and unique light source configurations for any product using a variety of light sources, such as visible light, xenon arc solar simulators, and fluorescent UV radiation sources. Other Our company services, such as pathology, pharmacokinetics, analytical and bioanalytical chemistry, clinical chemistry, quality assurance, and report production, reinforce our expertise in assessing phototoxic effects.

Phototoxicity Solutions
Our company offers a wide range of solutions for phototoxicity studies, including the following types
In Vitro Phototoxicology
Our company performs in vitro photosafety assessments, primarily in vitro 3T3 neutral red absorption phototoxicity tests. In addition to the 3T3 assay, we can also perform in vitro assessments of phototoxicity using the 3D epidermal cell system, photomutagenesis assay, and melanin binding assay. We will customize assays to screen a large number of compounds for compound selection during development while performing GLP-compliant assays.
In vitro phototoxicity tests
- In vitro 3T3 cell neutral red phototoxicity assay.
Cutaneous Phototoxicology
We perform phototoxicity analyses on mice, rats and guinea pigs, as well as photoallergy studies on guinea pigs. We also offer safety assessment studies to evaluate photodynamic drugs and unique light sources. Routes of administration include oral, intravenous, dermal, intraocular and ocular drips, including single and multiple doses.
In vivo phototoxicity tests (guinea pigs, rats, mice):
- Dermal phototoxicity test for in vivo administration and dermal administration in guinea pigs.
- Dermal phototoxicity test for in vivo and dermal administration in rats.
- In vivo and dermal phototoxicity test in mice.
- Dermal phototoxicity test in Brown Norway rats for in vivo and dermal administration.
- Dermal photoallergic reaction test in guinea pigs.
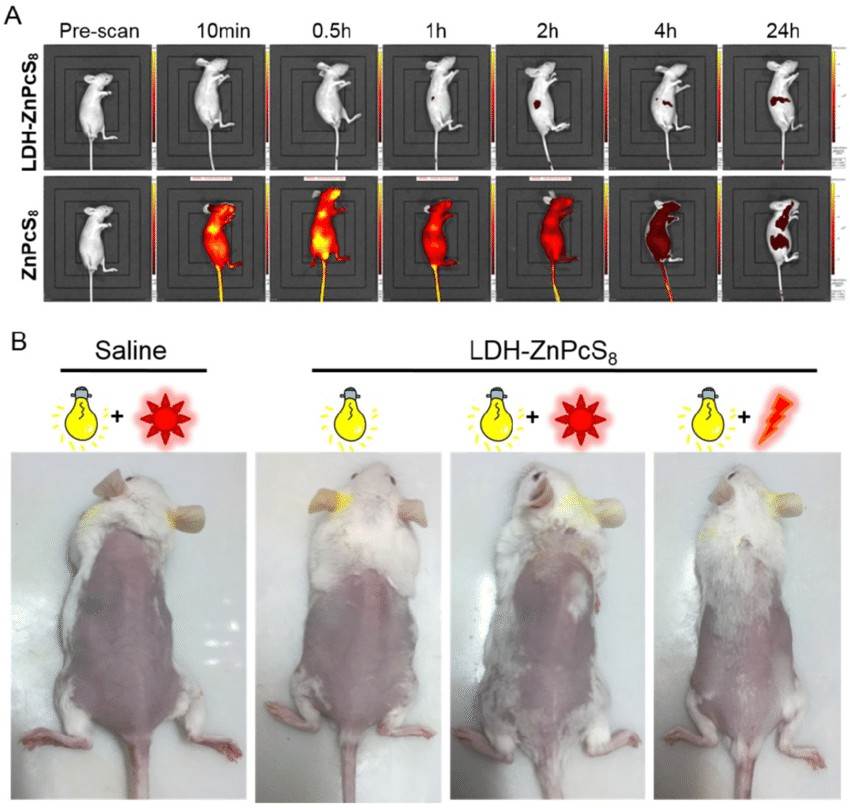 Fig.1 Detection of skin phototoxicity: (A) In vivo fluorescence imaging of mice before and after intravenous injection at different time points. (B) Photographs of mice after treatment with drugs under room light, sunlight and/or laser irradiation. (Li X, et al. 2017)
Fig.1 Detection of skin phototoxicity: (A) In vivo fluorescence imaging of mice before and after intravenous injection at different time points. (B) Photographs of mice after treatment with drugs under room light, sunlight and/or laser irradiation. (Li X, et al. 2017)
Ocular Phototoxicology
Our company has experienced veterinary ophthalmologists and pathologists who specialize in evaluating light-induced ocular pathology. We can assess how exposure to UV and visible light affects the ocular structures. We can use slit lamp biomicroscopy, indirect ophthalmoscopy, and intraocular pressure measurements in our study designs, which can also involve acute, continuous, or intermittent light exposure.
Visceral Phototoxicology
Our company may determine the possibility of harm to rodent thoracic and abdominal organs by evaluating light exposure during surgical procedures utilizing ultraviolet radiation (UVR), visible, or near-infrared light sources.
If you are looking for the best solution for the safety evaluation of drugs for human use, please feel free to contact us.
Reference
- Li X, et al. (2017). "A Tumor-pH-Responsive Supramolecular Photosensitizer for Activatable Photodynamic Therapy with Minimal In Vivo Skin Phototoxicity." Theranostics. 7(10): 2746-2756.
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 Detection of skin phototoxicity: (A) In vivo fluorescence imaging of mice before and after intravenous injection at different time points. (B) Photographs of mice after treatment with drugs under room light, sunlight and/or laser irradiation. (Li X, et al. 2017)
Fig.1 Detection of skin phototoxicity: (A) In vivo fluorescence imaging of mice before and after intravenous injection at different time points. (B) Photographs of mice after treatment with drugs under room light, sunlight and/or laser irradiation. (Li X, et al. 2017)