Developmental and reproductive toxicology (DART) studies are an important element of drug and chemical safety evaluation and are used to assess the reproductive safety of a drug, chemical or pesticide after repeated or prolonged exposure.
Our company has extensive experience in conducting a wide range of reproductive toxicology studies on animals such as rats and rabbits and can provide customers with a full range of reproductive and developmental toxicity tests. All tests are conducted in compliance with international GLP regulations.
Developmental and Reproductive Toxicology Studies
Years of accumulated historical control databases, a stable technical team and dedicated senior research staff ensure that Our company is able to provide high-quality services to our customers in a timely and efficient manner. We offer flexible assay designs for our customers' Phase I, II and III DART assays and can fully customize the assays to meet the specific needs of their projects.
Our safety evaluation team provides IND and NDA-supported DART studies in mice, rats and rabbits. We have tested a variety of rodent and non-rodent species with dosing routes ranging from dermal infusion to intravenous infusion. A range of specialized functional assessments is also available, including neurobehavioral testing and immunological assays.
All studies are supported by our own laboratories and regulatory experts and can all be conducted within the guidelines of international regulatory agencies.
Developmental and Reproductive Toxicology Solutions
Our company offers a wide range of DART test solutions to support our customers' DART studies.
- Embryo-fetus developmental dose range exploration assay.
- Fertility and early embryo-fetal development toxicity studies in rats and mice (Seg I).
- Embryo-fetal development toxicity test in mice, rats and rabbits (Seg II).
- Perinatal developmental toxicity test in rats, mice and rabbits (Seg III).
- Enhanced pre- and postnatal developmental Study (ePPND) in crab monkeys.
- Embryo-fetal developmental study (EFD) for crab-eating monkeys.
- Multi-generation reproduction study.
- Juvenile animal testing to support the safety evaluation of pediatric drugs.
- Male reproductive function studies.
- Sperm count, sperm motility, sperm morphology, histopathology.
- Evaluation of lactation and placental transit.
- Neurobehavioral tests (motor behavior, learning memory evaluation).
- Spontaneous mobility evaluation.
- Growth development and reflex function tests (growth hormone and insulin-like growth factor-1 tests, bone density and bone mass tests, shape differentiation, sex differentiation, reflex function).
- Evaluation of bone development.
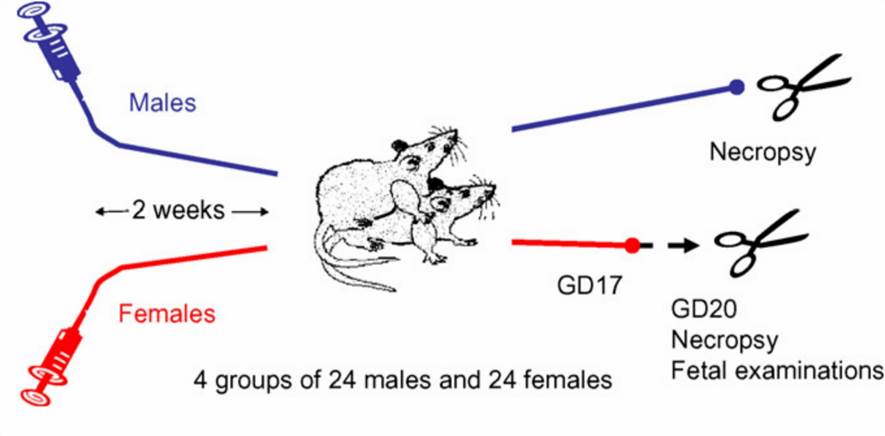 Fig.1 Combined fertility and embryotoxicity study design. GD=gestation day. (Barrow P. C, et al. 2009)
Fig.1 Combined fertility and embryotoxicity study design. GD=gestation day. (Barrow P. C, et al. 2009)
DART Study Options
- Routes of administration include oral, IM, IV, dermal, ocular and inhalation.
- Small molecule drugs, biologics, vaccines, chemicals, consumer products and agrochemicals.
If you are looking for the best solution for the safety evaluation of drugs for human use, please feel free to contact us.
Reference
- Barrow P. C, et al. (2009). "Reproductive Toxicity Testing for Pharmaceuticals Under ICH." Reproductive Toxicology. 28(2): 172-179.
Related Solutions
It should be noted that our service is only used for research, not for clinical use.


 Fig.1 Combined fertility and embryotoxicity study design. GD=gestation day. (Barrow P. C, et al. 2009)
Fig.1 Combined fertility and embryotoxicity study design. GD=gestation day. (Barrow P. C, et al. 2009)