 Kim, Min-Jeong, et al. Pharmacology & Pharmacy, 2011, 2(03), 189-193.
Kim, Min-Jeong, et al. Pharmacology & Pharmacy, 2011, 2(03), 189-193.
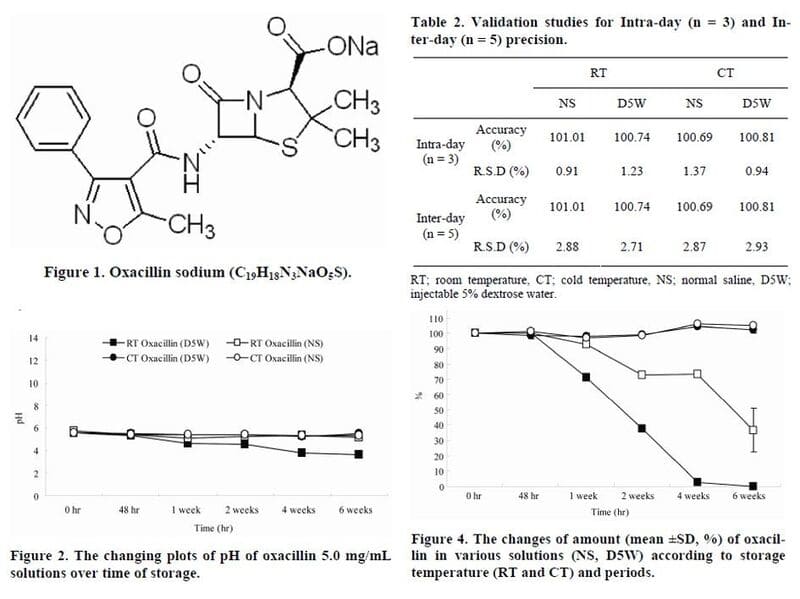
Two commonly used oxacillin sodium solutions, 0.9% sodium chloride (NS) or 5% dextrose in water (D5W) at a concentration of 5.0 mg/mL, were compared for suitability for intravenous administration after six weeks of storage at two different controlled temperatures and for the stability of the active compound over time.
· Evaluation methods
Both solutions were stored in commercially available intravenous infusion sets and maintained continuously at 4 ± 2°C (CT) or 25 ± 2°C (RT). Suitability for intravenous administration was evaluated by measuring changes in macroscopic transparency and pH over time, and drug stability was evaluated by measuring changes in oxacillin concentration over time using high-performance liquid chromatography (HPLC).
· Results
After 6 weeks, oxacillin concentrations in the CT solution remained unchanged, while oxacillin concentrations in both RT solutions decreased significantly after only two weeks. After 6 weeks at room temperature, the final concentration in NS was 36.57% compared to the starting concentration, while oxacillin was barely detectable in D5W.
In addition, pH measurements showed a slight decrease in pH at 2 weeks at room temperature, and significant changes in pH at 6 weeks in both NS and D5W. None of these oxacillin solutions showed significant changes in color, clarity, or appearance after 6 weeks.
Overall, ready-to-use oxacillin sodium solutions in infusion sets can be safely stored at CT for up to 6 weeks but should not be stored at room temperature due to loss of potency and changes in pH.


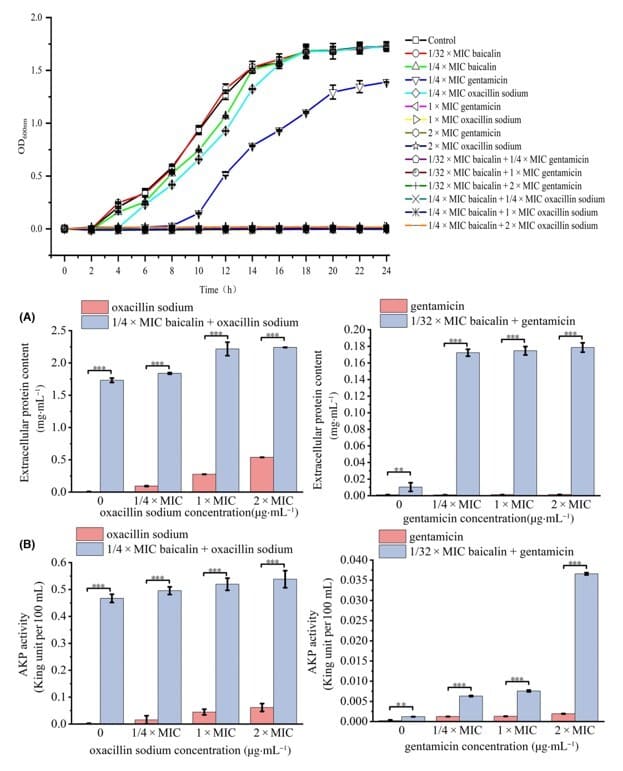 Meng, Xin, et al. FEBS Open Bio., 2024.
Meng, Xin, et al. FEBS Open Bio., 2024.
 Kim, Min-Jeong, et al. Pharmacology & Pharmacy, 2011, 2(03), 189-193.
Kim, Min-Jeong, et al. Pharmacology & Pharmacy, 2011, 2(03), 189-193.